New crystal structures of translocating ribosome
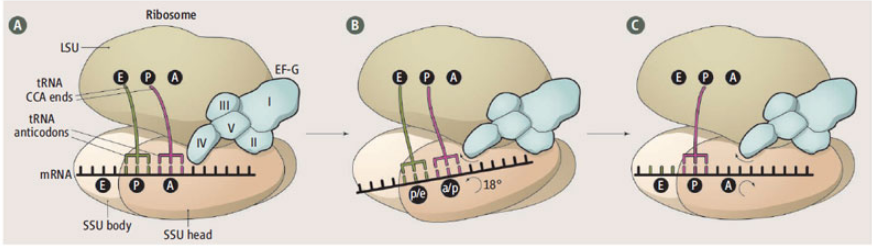
Several new crystal structures of the 70S ribosome in complex with EFG and non-hydrolyzable GTP analogs have revealed how the ribosome directionally translocates mRNA and the tRNAs through the A, P, and E sites and how specific features of EFG and ribosomal RNA act as pawls to enforce this ratcheting mechanism. The new structures were solved in the Cate, Noller, and Ramakrishnan labs and were published in the June 28 edition of Science. Many of the new structures were made possible by data collected at the SIBYLS beamline.
If you want the boiled down version of these new results then read this succinct comment by Marina Rodnina.
A figure liberally lifted from her comment.
