How a Shape-shifting DNA-repair Machine Fights Cancer
The SIBYLS beamline was instrumental in providing key structural data for two recent publications exploring the dynamic nature of DNA repair.

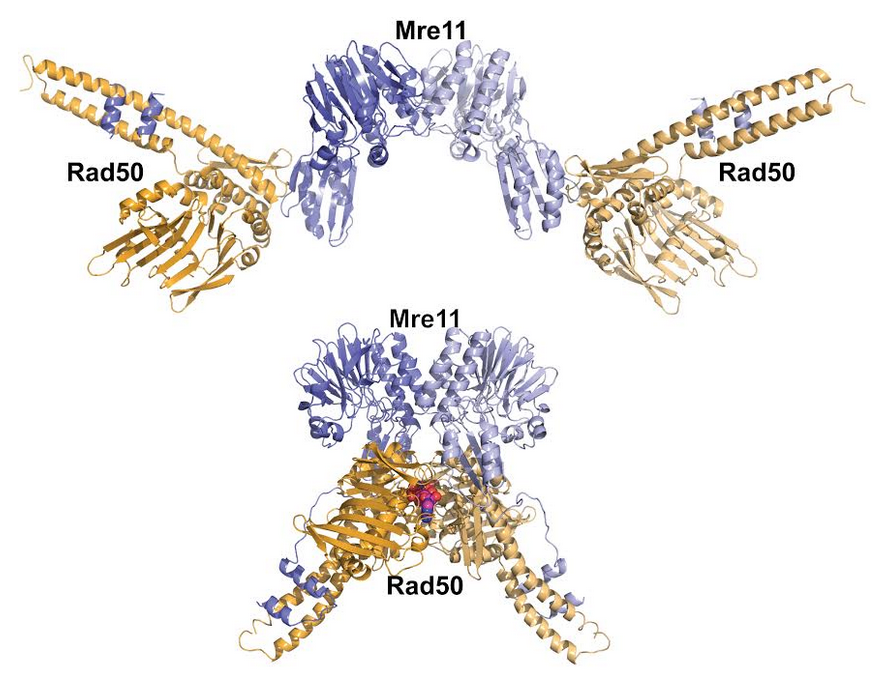
>The Mre11‐Rad50 complex is highly conserved, yet the mechanisms by which Rad50 ATP‐driven states regulate the sensing, processing and signaling of DNA double‐strand breaks are largely unknown. Here we design structure‐based mutations in Pyrococcus furiosus Rad50 to alter protein core plasticity and residues undergoing ATP‐driven movements within the catalytic domains. With this strategy we identify Rad50 separation‐of‐function mutants that either promote or destabilize the ATP‐bound state. Crystal structures, X‐ray scattering, biochemical assays, and functional analyses of mutant PfRad50 complexes show that the ATP‐induced ‘closed’ conformation promotes DNA end binding and end tethering, while hydrolysis‐induced opening is essential for DNA resection. Reducing the stability of the ATP‐bound state impairs DNA repair and Tel1 (ATM) checkpoint signaling in Schizosaccharomyces pombe, double‐strand break resection in Saccharomyces cerevisiae, and ATM activation by human Mre11‐Rad50‐Nbs1 in vitro, supporting the generality of the P. furiosus Rad50 structure‐based mutational analyses. These collective results suggest that ATP‐dependent Rad50 conformations switch the Mre11‐Rad50 complex between DNA tethering, ATM signaling, and 5′ strand resection, revealing molecular mechanisms regulating responses to DNA double‐strand breaks.
read more in the full articles…
ATP-driven Rad50 conformations regulate DNA tethering, end resection, and ATM checkpoint signaling.” EMBO J. 2014 Feb 3.
and