Protein flexibility linked to severity of Lou Gerhig’s disease
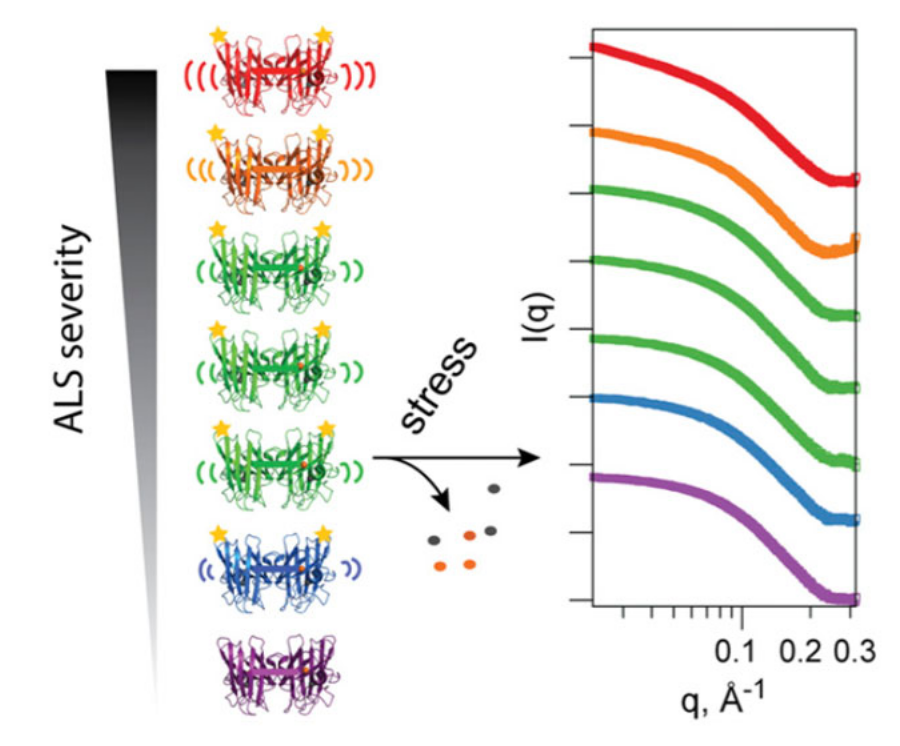
SAXS and MX data collected at SIBYLS enabled insights into protein flexibility in one of the proteins involved in Lou Gerigh’s disease. The results were recently published in PNAS and have been highlighted by [Today At Berkeley Lab](http://newscenter.lbl.gov/2014/10/13/berkeley-lab-and-scripps-research-institute-scientists-link-als-progression-to-increased-protein-instability/) and and [Daily Cal](http://www.dailycal.org/2014/10/16/berkeley-lab-scripps-institute-propose-cause-als/).
>Protein framework alterations in heritable Cu, Zn superoxide dismutase (SOD) mutants cause misassembly and aggregation in cells affected by the motor neuron disease ALS. However, the mechanistic relationship between superoxide dismutase 1 (SOD1) mutations and human disease is controversial, with many hypotheses postulated for the propensity of specific SOD mutants to cause ALS. Here, we experimentally identify distinguishing attributes of ALS mutant SOD proteins that correlate with clinical severity by applying solution biophysical techniques to six ALS mutants at human SOD hotspot glycine 93. A small-angle X-ray scattering (SAXS) assay and other structural methods assessed aggregation propensity by defining the size and shape of fibrillar SOD aggregates after mild biochemical perturbations. Inductively coupled plasma MS quantified metal ion binding stoichiometry, and pulsed dipolar ESR spectroscopy evaluated the Cu2+ binding site and defined cross-dimer copper-copper distance distributions. Importantly, we find that copper deficiency in these mutants promotes aggregation in a manner strikingly consistent with their clinical severities. G93 mutants seem to properly incorporate metal ions under physiological conditions when assisted by the cop- per chaperone but release copper under destabilizing conditions more readily than the WT enzyme. Altered intradimer flexibility in ALS mutants may cause differential metal retention and promote distinct aggregation trends observed for mutant proteins in vitro and in ALS patients. Combined biophysical and structural results test and link copper retention to the framework destabilization hypothesis as a unifying general mechanism for both SOD aggregation and ALS disease progression, with implications for disease severity and therapeutic intervention strategies.

Pratt AJ, Shin DS, Merz GE, Rambo RP, Lancaster WA, Dyer KN, Borbat PP, Poole FL, Adams MW, Freed JH, Crane BR, Tainer JA, Getzoff ED. “Aggregation propensities of superoxide dismutase G93 hotspot mutants mirror ALS clinical phenotypes.” Proc. Natl. Acad. Sci. U.S.A. 2014 Oct 14 link