Defining NADH-Driven Allostery Regulating Apoptosis-Inducing Factor
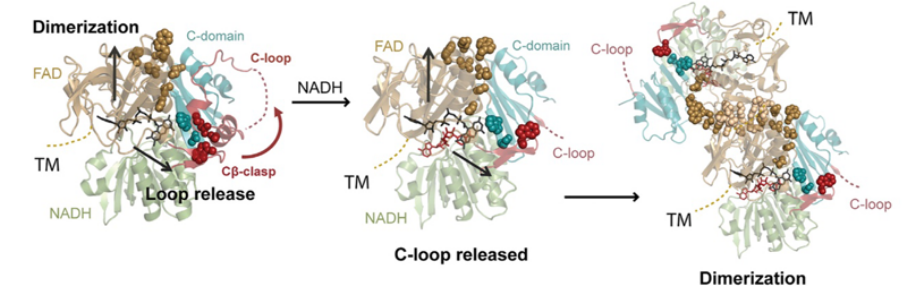
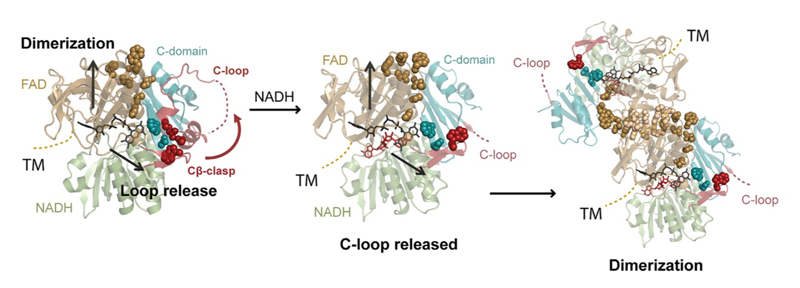
Apoptosis-inducing factor (AIF) is critical for mitochondrial respiratory complex biogenesis and for mediating necroptotic parthanatos; these functions are seemingly regulated by enigmatic allosteric switching driven by NADH charge-transfer complex (CTC) formation. In this paper the authors define molecular pathways linking AIF’s active site to allosteric switching regions by characterizing dimer-permissive mutants using small-angle X-ray scattering (SAXS) and crystallography and by probing AIF-CTC communication networks using molecular dynamics simulations. Collective results identify two pathways propagating allostery from the CTC active site: (1) active-site H454 links to S480 of AIF’s central β-strand to modulate a hydrophobic border at the dimerization interface, and (2) an interaction network links AIF’s FAD cofactor, central β-strand, and Cβ-clasp whereby R529 reorientation initiates C-loop release during CTC formation. This knowledge of AIF allostery and its flavoswitch mechanism provides a foundation for biologically understanding and biomedically controlling its participation in mitochondrial homeostasis and cell death.