Designing and defining dynamic protein cage nanoassemblies in solution
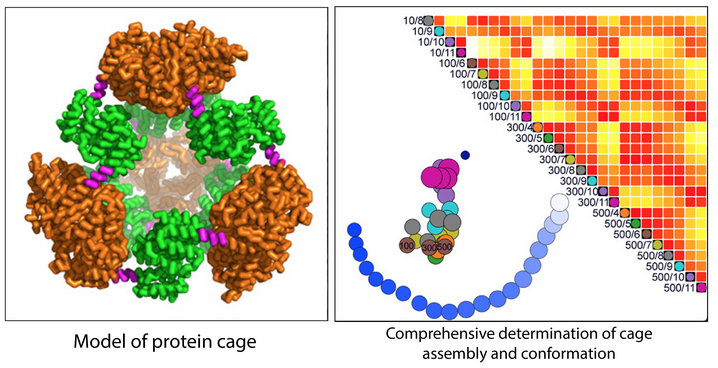

Central challenges in the design of large and dynamic macromolecular assemblies for synthetic biology lie in developing effective methods for testing design strategies and their outcomes, including comprehensive assessments of solution behavior. The authors of this paper created and validated an advanced design of a 600-kDa protein homododecamer that self-assembles into a symmetric tetrahedral cage. The monomeric unit is composed of a trimerizing apex-forming domain genetically linked to an edge-forming dimerizing domain. Enhancing the crystallographic results, high-throughput small-angle x-ray scattering (SAXS) comprehensively contrasted our modifications under diverse solution conditions. To generate a phase diagram associating structure and assembly, we developed force plots that measure dissimilarity among multiple SAXS data sets. These new tools, which provided effective feedback on experimental constructs relative to design, have general applicability in analyzing the solution behavior of heterogeneous nanosystems and have been made available as a web-based application. Specifically, our results probed the influence of solution conditions and symmetry on stability and structural adaptability, identifying the dimeric interface as the weak point in the assembly. Force plots comparing SAXS data sets further reveal more complex and controllable behavior in solution than captured by our crystal structures. These methods for objectively and comprehensively comparing SAXS profiles for systems critically affected by solvent conditions and structural heterogeneity provide an enabling technology for advancing the design and bioengineering of nanoscale biological materials.
Yen-Ting Lai1, Greg L. Hura, Kevin N. Dyer, Henry Y. H. Tang, John A. Tainer and Todd O. Yeates [Designing and defining dynamic protein cage nanoassemblies in solution](http://advances.sciencemag.org/content/2/12/e1501855.full) 14 Dec 2016:Vol. 2, no. 12