Scaffold protein compacts human DNA Ligase
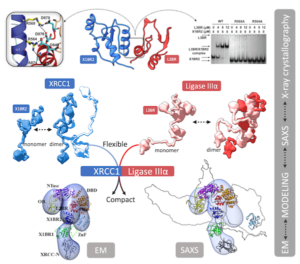
SIBYLS scientist, Michal Hammel, analyzing data collected at the SIBYLS beamline gained insight into the shape and conformational flexibility of the XL as well as XRCC1 and DNA ligase IIIα (LigIIIα) alone. This ligase complex is critical for DNA single-strand break repair, a key target for PARP inhibitors in cancer cells deficient in homologous recombination. These results helped identify an atypical BRCT-BRCT interaction as the stable nucleating core of the XL that links the flexible nick sensing and catalytic domains of LigIIIα to other protein partners of the flexible XRCC1 scaffold.