SAXS study of radiation damage
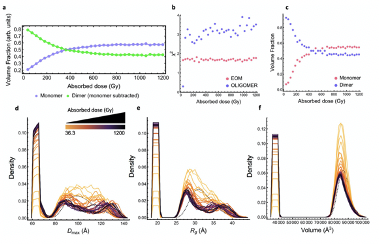
SIBYLS user Dr. Tim Stachowski from the Snell group at Hauptman-Woodward Medical Research Institute, led a research effort combining a protein engineering approach with SAXS to monitor cleavage of a specific bond from exposure to the X-ray beam. X-ray induced disulfide bond breakage is a common phenomenon in X-ray crystallography but there is limited information on how susceptible disulfides are to low X-ray doses and in solution. To detect disulfide bond breakage with a low-resolution technique like SAXS, a protein was coerced to dimerize through a susceptible disulfide bond. During X-ray exposure, breakage of the bond was apparent by monitoring how the protein sample transitioned from a dimer to a monomer using several metrics calculated directly from the SAXS data. These findings are an important step towards understanding if a connection can be made between the detailed crystallographic radiation damage mechanisms and the solution state, which is closer to physiology, as well as understanding how samples can potentially be altered during the course of SAXS experiments.